Let our team of digital laboratory leaders and medical device regulation experts help you understand your obligations, assess your gaps, build a roadmap, and get compliant across your information systems.
Together, we'll develop a path to compliance aligned with the phase-out approach.
LEAP INTO FDA LDT FINAL RULE COMPLIANCE
EXPLORE OUR PHASED APPROACH
Leap offers hands-on support for adaptation of your existing CLIA, CAP, and NYS LIS and LIMS systems, policies, and SOPs to achieve FDA LDT Final Rule compliance in accordance with the published stages of enforcement discretion, including CFR 21 Part 11 and more immediately subpart-m compliance for record keeping supporting adverse events and related reporting.
-
INTRODUCTORY CHANGE SCOPE INVENTORY
Let us develop a list of your systems and processes for audit and remediation.
-

FULL FINAL RULE GAP ANALYSIS
Get an assessment of your systems, compliance documents, and more to ensure compliance.
-

ROADMAP TO READINESS
Receive support from inventory to gap analysis, with collaboration to adapt solutions.
-

DESIGN YOUR COMPLIANCE PROGRAM
Custom engagements include audits, roadmapping, remediation, recommendations, and solutions.
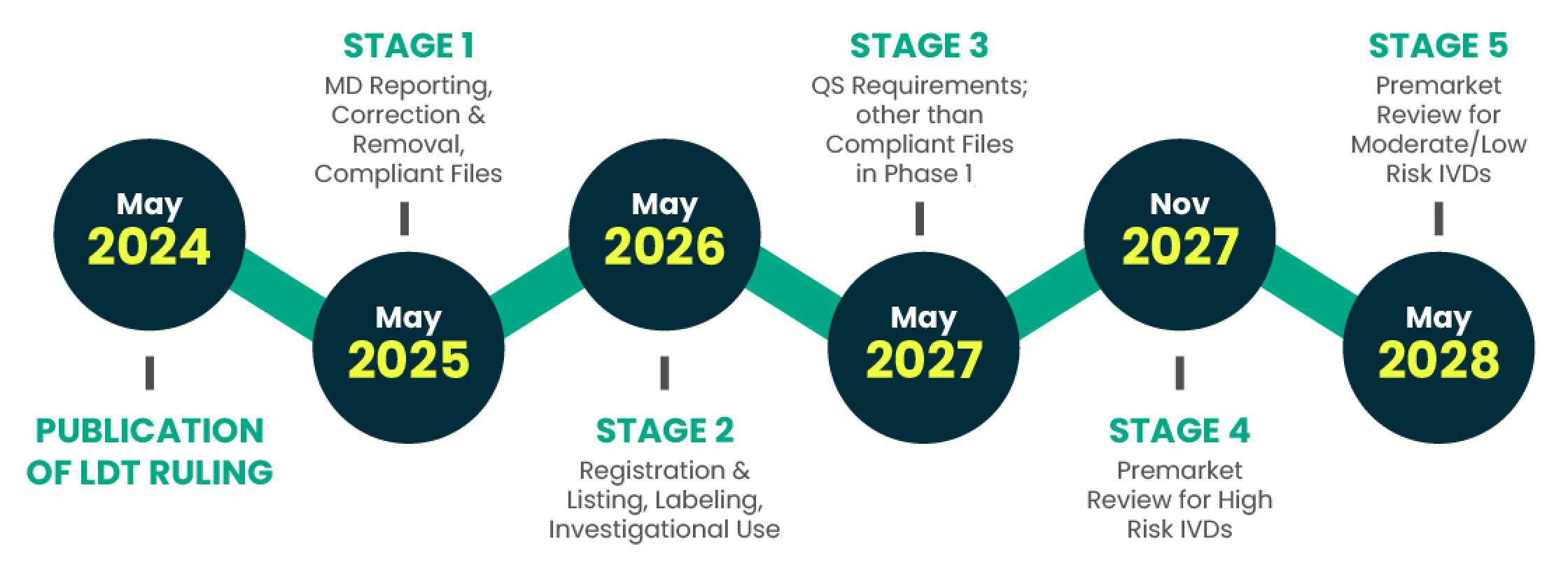
FDA ENFORCEMENT DISCRETION TIMELINE
The FDA's April 2024 ruling outlines an enforcement timeline. Identifying compliance gaps, effort, and needed resources is crucial for budgeting and meeting deadlines.
How else can we help you accelerate, simplify, and better digitize your business?
LEAP IS READY TO SUPPORT YOUR FDA COMPLIANCE
Learn more about the FDA's April 2024 ruling on LDT regulation and discover our approach for labs already complying with CLIA, CAP, and NYS regulations.
THE TIME IS NOW
The FDA's April 2024 ruling offers a timeline for enforcement discretion, which can be changed at any time. Understanding the major gaps in your compliance posture, your level-of-effort to comply, and the resources required are all key to ensuring you are able to budget accordingly and meet the deadlines.
Act immediately to Identify current compliance gaps.
Establish a roadmap in 2024, including a detailed action plan with deadlines for compliance.
Stage 1, compliant completion, retention, and reporting of associated documentation, is due May 6, 2025
Stages 2 through Stage 5 continue through 2028, including Quality System enforcement deadlines in 2027.
Plan for change in technology, processes, and documentation across the lab(s).
Policy Timeline
Security
Input/data capture
Audit Trail
Electronic signature
Archival & storage
Search & retrieval
Change control
Long-term preservation & access
Reporting
Technology Requirements
Training & Education
Policies
Procedures
Business Process
Documentation
Reporting
Process Requirements
A TRUSTED PARTNER
Leap brings extensive expertise in diagnostics, technology, process design, implementation, and FDA compliance to transition seamlessly to meet FDA LDT medical device compliance obligations building on your existing systems and processes.
Clinical and Biopharma
Trusted partner for biotech, biopharma, laboratory, and healthcare organizations since 2012.
Extensive track record of assessing, designing, developing, and scaling compliant laboratory information systems throughout the United States.
Experience developing compliance with FDA, GXP, and related regulatory standards.
Experienced Consultants
Business people, analysts, and engineers who understand laboratory medicine.
Deep understanding of the unique challenges of operating in regulated environments.
Collaborative approach to navigating the complex journey of building products and services using a best-of-breed approach to buy and build right-sized solutions.
Partners at Scale
Decades of experience building robust technology platforms, building partnerships, and tackling complex challenges in growth and transformation.
Trusted, durable solutions built on expansive thinking, a disciplined process framework, and impeccable service.
FDA LDT FINAL RULE READINESS
Planning is essential to prioritize system and process changes to meet your FDA obligations.
Information Request
Summary of current lab systems, electronic records, change control, and reporting procedures.
Technology Analysis
Assess systems for open/closed status to identify required security protocols.
Gap Analysis of Processes
Identify and analyze compliance gaps in current processes and systems.
Action Plan
Create an action plan for system configurations, policy updates, and business process improvements aligned to a timeline with resourcing.
Remediation and Adaptation
Engage teams to implement changes, create policies, and conduct training sessions.
PLAN PACKAGES
From lab transformation to FDA compliance, LEAP advises on people, processes, and platforms as well as full-lifecycle technology implementation services.
A. CHANGE SCOPE ADVISORY ENGAGEMENT
Basic analysis of the organization's existing CLIA/CAP/NYS compliance & supporting documentation and systems.
LEARN MORE
Typical Project Length:
2 weeks
Detailed analysis of existing SOPs, checklists, systems, diagrams, and other compliance documentation provided against Part 11 requirements.
B. FULL FINAL RULE GAP ANALYSIS
Typical Project Length:
1-2 months
Full program support inclusive of A+B as well as collaborating with the organization to remediate and adapt documentation, processes, and systems.
LEARN MORE
C. ROADMAP TO FDA READINESS
Typical Project Length:
3 months
Fully customized support from high-level audits, to in-depth analysis and roadmapping, hands-on remediation, additional recommendations, and bespoke solutions.
LEARN MORE
D. CUSTOM PLANNING AND SUPPORT PROGRAM
Typical Project Length:
Discussion Required